Ester amidation the sodium methoxide way
22 April 2012 - Orgo
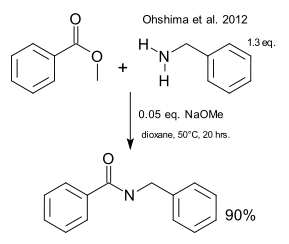 Amides are important in organic chemistry but their synthesis involves more than a simple reaction between a carboxylic acid and an amine due to the unfavourable chemical equilibrium. In a variation called the Schotten-Baumann reaction the acid is activated as an acid chloride. You will not find esters in any list of amide precursors but a report by Ohshima et al. suggests esters and amines can react directly although with an unlikely catalyst: sodium methoxide ( DOI).
Amides are important in organic chemistry but their synthesis involves more than a simple reaction between a carboxylic acid and an amine due to the unfavourable chemical equilibrium. In a variation called the Schotten-Baumann reaction the acid is activated as an acid chloride. You will not find esters in any list of amide precursors but a report by Ohshima et al. suggests esters and amines can react directly although with an unlikely catalyst: sodium methoxide ( DOI).
In one exploit the reagents are methyl benzoate and benzylamine and in dioxane the yield with just 5% NaOMe is 90%. The only catch is that the protocol requires absolute exclusion of water because otherwise the only thing that happens is ester saponification. Other bases that work are sodium tert-butoxide and NaHMDS. Bases that do not work are lithium tert-butoxide or lithium bis(trimethylsilyl)amide
