Dehydrocoupling
5 December 2008 - Synthetic methods
What: Dehydrocoupling is the formation of a new main group metal-metal chemical bond with formation of hydrogen gas from a hydride with a suitable catalyst (recent review DOI). A type of dehydrogenation. First observed in dimerization of pentaborane by action of Platinum(II) bromide (Corcoran & Sneddon 1984 DOI). First silicon bond formation with (Aitken et al 1985 DOI) polymerization of phenylsilane in presence of the Petasis reagent to polyphenylsilane (DP around 10).
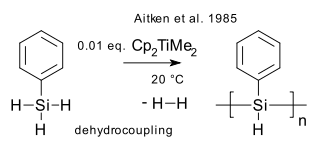
In a similar way polystannanes of the type (RR'Sn)n can be formed (Tilley et al. 1995 DOI).
Reaction of phenylphosphine with organozirconium catalyst Cp*2ZrCl2 and KH gives the cyclic pentamer (Stephan et al. 1995 DOI).
Mechanism: a sigma bond metathesis reaction mechanism is postulated for the polysilane formation reaction.
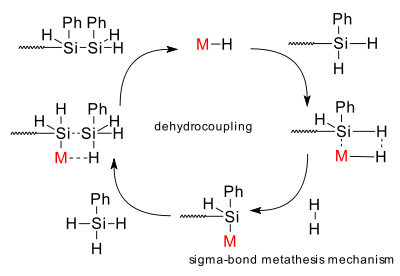
Also known as: dehydrogenative coupling - dehydropolymerization
Preceded by: Wurtz-Fittig reaction , coupling of elements through the halide and not the hydride. Disadvantages : use of expensive halides, handling of alkali metals.
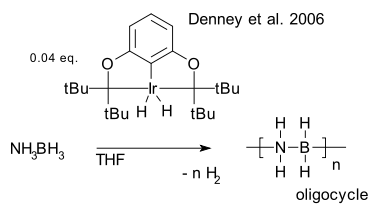
Scope: One particular dehydrocoupling is in vogue as hydrogen storage solution: the reaction of amino borane (Denney et al. 2006 DOI) with an organoiridium pincer catalyst to an cyclic aminoborane oligomer with quantitative formation of hydrogen.
:
Most recently in 2008 using the same catalyst a practical synthesis was reported for linear high molar mass poly(N-methylaminoborane) (NHMeBH2)n (Staubitz et al. DOI).
