Chemistry goes solar
08 February 2010 - green chemistry?
 Organic photochemists are taking the next logical step and venture out of their laboratory and into the sunshine. Obviously all photochemists require light do to their work but some of them shun artificial light bulbs and insist on the natural thing: solar light! And that all on account of greenness. Does this sound like some lame excuse for northern-hemisphere chemists to relocate to a sunnier climate?
Organic photochemists are taking the next logical step and venture out of their laboratory and into the sunshine. Obviously all photochemists require light do to their work but some of them shun artificial light bulbs and insist on the natural thing: solar light! And that all on account of greenness. Does this sound like some lame excuse for northern-hemisphere chemists to relocate to a sunnier climate?
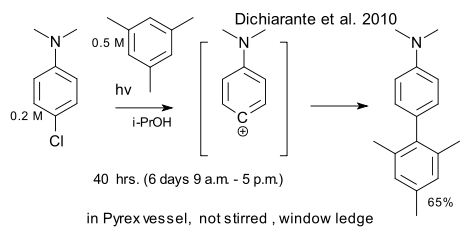
Italian chemists have recently invented the word solarylation for their solar powered aryl SN1 reactions that turn out to work just as well in sunlight as with artificial light sources (Dichiarante et al. DOI). All they had to do was switch to greener solvents and increase concentration. Besides mesitylene other suitable nucleophiles for this particular reaction are alkenes.
Scale-up of sun-powered reactions does not need to be an issue. Chemists from Ireland and Germany enthusiastically described the merits of solar light driven reactions in a 2007 article (DOI). Near Cologne, at the German Aerospace Center the Parabolic Trough Facility for Organic Photochemical Synthesis (PROPHIS) equipped with solar tracker has converted citronellol in an photooxygenation to an industrially important intermediate of rose oxide on a 8 liter scale with 70 liter of isopropanol with 2.5 hours exposure time (32 square meters surface area) and 133 moles of photons.
But is this really a viable contribution to green chemistry? From an economics point of view it is still smarter to use green solar electricity to power that light bulb. On the other hand in one particular branch of organic reactions - degradation - the merit is certainly there. Solar detoxification would be an interesting low-cost option for cleaning up remote but sunny contaminated water systems.
