Chemical Zoo: C2
04 May 2020 - Department of improbable molecules
 The synthesis of C2 has been reported by Miyamoto et al. (DOI) This long elusive chemical compound (impossible to make!) has a long history and even an history in this blog. In 2012 it was mentioned in connection to computations reported by Sason Shaik et al. (link) and in 2013 the blog gave a summary on an actual fight between several prominent chemists on the exact nature of bonding (link). Is the bond order two, three or four?
The synthesis of C2 has been reported by Miyamoto et al. (DOI) This long elusive chemical compound (impossible to make!) has a long history and even an history in this blog. In 2012 it was mentioned in connection to computations reported by Sason Shaik et al. (link) and in 2013 the blog gave a summary on an actual fight between several prominent chemists on the exact nature of bonding (link). Is the bond order two, three or four?
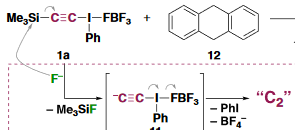
Perhaps they should have focused their energy instead on actually trying to make the compound. The new synthesis is an actual synthesis, not a few molecules captured by ATM, transient in a spectroscopic fly-by or embedded by huge ligands. No, the report speaks of actual C2 in a bottle, as a gas and at room-temperature. Even took them by surprise. Their success strategy was an acetylene with two unusuall leaving groups: Me3Si-C:::C-I(Ph)-BF4. In a reaction with TBAF at -78°C this compound was found to lose trimethylsilyl fluoride, phenyl iodide and tetrabutylammonium tetrafluorborate after warming-up to -30°C, leaving naked C2 by elimination. The authors point out that if a triflate is a super-leaving group then the phenyl-lambda-3-iodanyl group is a hyper-leaving group. Based on reactivity patterns the authors also conclude the compound is a singlet biradical. They do not mention bond order but a biradical would make the bond order three. C2 abstracts two protons from dihydroanthracene, it also reacts as a radical with radical scavenger galvinoxyl.
The report contains a second surprising follow-up reaction. When the precursor was ground with caesium fluoride in a mortar and pestle at room temperature the resulting soot was found to contain quantities of nanotubes and C60 molecules. This opens up a future for C2 as a common building block.
Rik
