Asymmetric photochemistry IV
10 july 2008 - Asymmetric photosensitization
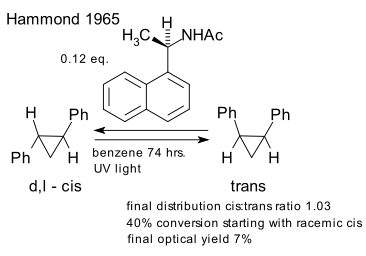
Hammond et al. in 1965 introduced asymmetric reactions involving a chiral photosensitizer (DOI). Racemic trans-diphenylcyclopropane was subjected to non-polarized UV radiation in presence of a chiral amide converting it to the achiral (meso) cis isomer up to 40%. The optical rotation of the remaining trans isomer was found to increase from 0 to 28° (7% optical yield). This effect is attributed to the formation of an excimer complex allowed transfer of chirality from the sensitizer to the reaction product.
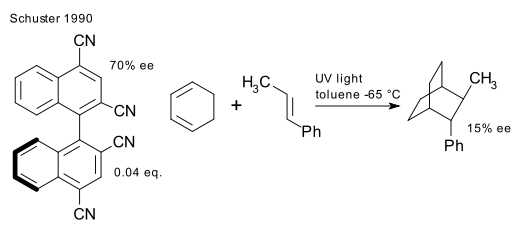
Optical yields were improved by Inoue et al. in 1989(DOI) and Schuster et al. in 1990 ( DOI)
Inoue photoisomerized cis-cyclooctene to the trans isomer (chiral) with 12% optical purity with a photosensitizer based on chiral borneol. Schuster's system is based on a Diels-Alder reaction between beta-methylstyrene and cyclohexadiene and a complex Binaphthalene sensitizer.