Asymmetric beta C-H activation
24 September 2016 - Organic reactions
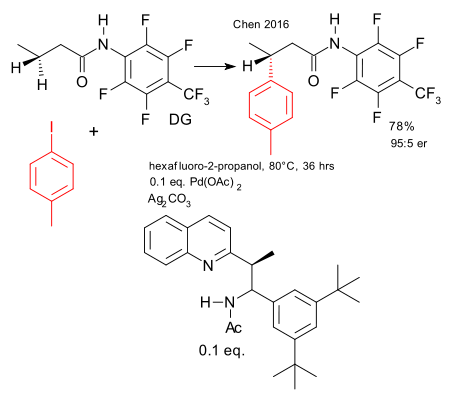 A recent contribution from the Kendall Houk laboratory concerns the asymmtric version of a palladium catalysed beta C-H-activation (Gang Chen et al. DOI). This work builds on work done by Giri et al. in 2005 on asymmetric Pd iodination (DOI).
A recent contribution from the Kendall Houk laboratory concerns the asymmtric version of a palladium catalysed beta C-H-activation (Gang Chen et al. DOI). This work builds on work done by Giri et al. in 2005 on asymmetric Pd iodination (DOI).
The substrates are amides with unactivated protons at the beta position. The large fluorinated aryl group acts as a directing group. The catalyst system is palladium acetate and a custom-built bulky chiral aminoethyl quinoline ligand (isoquinoline plus N-Sulfinyl imine chemistry). In the transition state palladium is coordinated to all three nitrogen atoms with remaining coordination site interacting with the beta methylene group. Yield 78%, enantiomeric ratio 95:5.
