A new tail for Taxol
22 March 2009 - news
A team of Sweden-based chemists (DOI) have come up with a new way to add the tail in the cancer-curing drug Paclitaxel or Taxol (if you feel companies should not be allowed to hijack generic names as trade names). Although Taxol is a biomolecule that can be harvested from Taxus brevifolia, more of it is produced commercially from tail-less baccatin III that can be extracted from the more handy Taxus baccata. The tail is then added by reaction with the Ojima lactam (see Taxol total synthesis).
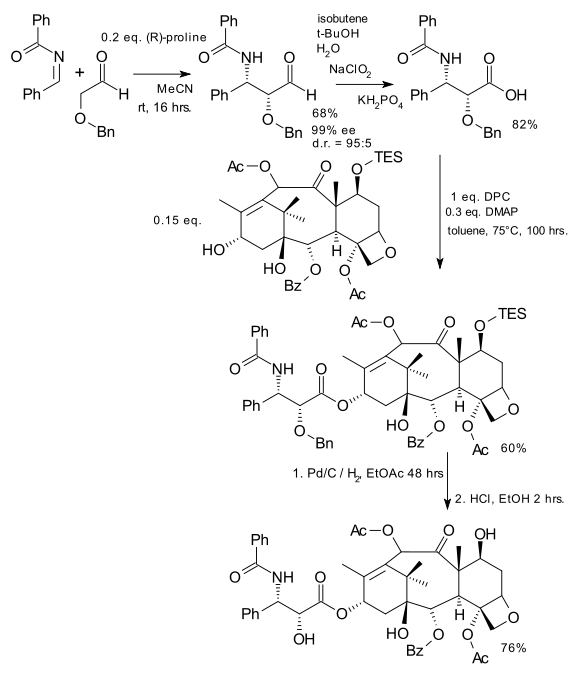
The new method which appears less of a hassle than the Ojima method is based on an asymmetric Mannich reaction catalysed by (R)-proline. The aldehyde group is the oxidized to a carboxylic acid using Sodium chlorite and for some reason isobutene and KH2PO4 are thrown in as well. Esterfication to TES-protected baccatin III is accomplished with DMAP and DCP (di-2-pyridyl carbonate) and final deprotection with palladium on carbon and hydrochloric acid.